Did you know that the global pharmaceutical market is worth around $1228.45 and growing every day?
The world of pharma is elusive to most, and it can be challenging to sort facts from fiction. In pandemic times with controversy at every turn, who really knows what goes on within pharmaceutical companies?
That’s where our helpful guide comes in; we’re here to tell you the facts. Next time pharmaceuticals come up in conversation, you’ll know exactly what’s true and what isn’t.
If this interests you, read on to find out more.
1. Profits From Prescriptions Decreased During COVID-19
Contrary to what you might think, there was actually less demand for prescriptions during the pandemic.
There are several reasons for this drop in demand. Firstly, healthcare anxiety at the start of the pandemic put-off non-emergency appointments. Therefore, with fewer GP appointments came fewer prescriptions.
Customer confidence in pharmaceutical companies has also reduced. As a result, people have been turning to more natural treatment methods.
However, many of these companies now have excess pharmaceutical stocks ready to provide in 2022.
2. Pharma Provides Millions of Jobs
You may think that only scientists and high-level executives work in pharma. The reality is very different; the pharmaceutical industry provides millions of jobs, from lab technicians to pharmacologists.
Some of the main pharma jobs include:
- Pharma sales rep
- Clinical data manager
- Research scientist
- Pharmacy manager
- Biostatistician
AstraZeneca alone provides around 50,000 pharmaceutical sales jobs. Working for pharmaceutical companies can be incredibly lucrative. Roles in corporate offices and sales often pay upwards of £60,000 and include performance-related bonuses.
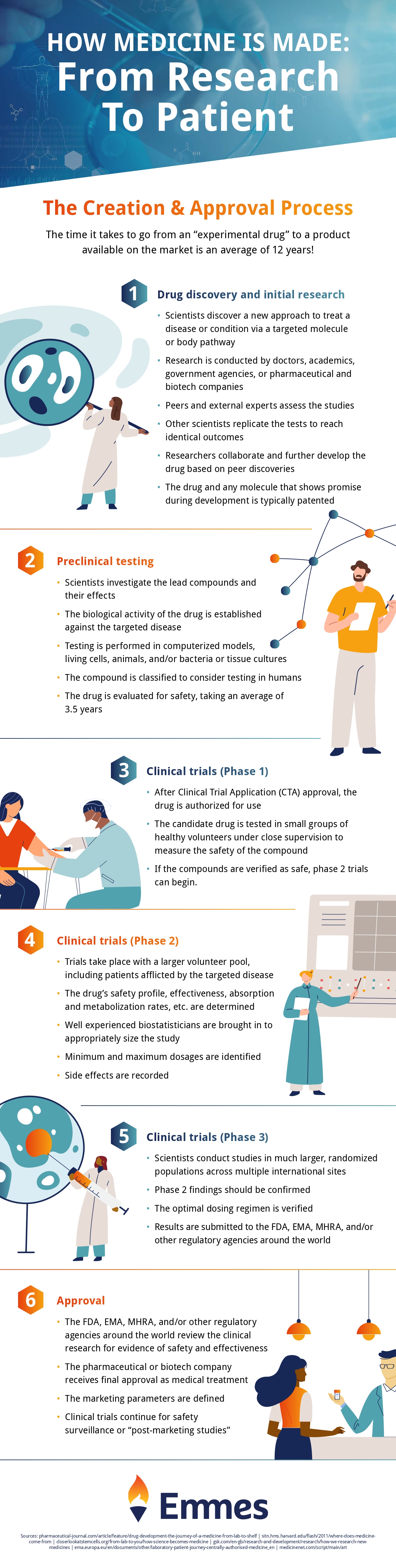
3. Making Drugs Is a Lengthy Process
To ensure medications are safe, they must undergo pharmaceutical testing in several trials. In fact, on average it takes over ten years to bring a new drug to market.
Once the medicine has been researched, discovered, and synthesized, there are four stages of drug development.
In the first phase, researchers determine whether or not a medicine is safe for human usage. It entails recruiting a limited number of healthy volunteers who will be given single treatment doses. They’re observed, and feedback is gathered to see how the medicine affects them.
After a drug’s safety has been established, it must be evaluated how successful it is in curing or preventing disease on a larger scale. A bigger sample size of roughly 100 or 200 volunteers is usually utilized for a controlled, randomized, or double-blind study. This study may last months or years.
Once it’s passed stages one and two, a new drug can progress to the third phase. This involves repeating the same types of clinical trials on a larger and broader scale.
This trial will need thousands of patients. Stage three takes several years to complete and provides more insight into how the drug affects different people over time.
Finally, evidence from all three previous steps is compiled and evaluated in stage four. It’s then presented to the Medicines and Healthcare Products Regulatory Agency in the United Kingdom (MHRA). The MHRA will license the drug if the evidence shows that it is both effective and safe for human consumption.
There are entire companies dedicated to managing clinical trials supply issues and ensuring that large-scale clinical trials run smoothly.
4. There Were Supply Chain Disruptions In 2021
Although global pharmaceutical supply chains did not collapse due to COVID-19, the pandemic exposed flaws in pharma logistics.
The industry’s efficient supply chains are not immune to production challenges caused by events like a pandemic. As a result, extended production lead times and fluctuating demand posed issues throughout 2021.
Although some companies have made up for lost profits through vaccine manufacturing, there were undoubtedly many unseen costs for these companies in 2021.
5. Pharmaceutical Fraud Is a Problem
Pharmaceutical fraud is still a serious issue for the business, and it may have gotten much worse during COVID-19. Misconduct during the epidemic could result in higher-than-average healthcare fraud recoveries this year.
Fraud happens through several avenues. The first problem is counterfeit drugs being sold and taking profits from pharma companies. However, this only has a small impact. More significant threats come from hackers and data breaches.
Additionally, insurance fraud can have a domino effect and also affect the pharma industry.
Protective regulations helped decrease the amount of fraud that happened during the pandemic. However, huge recoveries still need to be made up. This may significantly disrupt the business as a whole throughout 2022.
Manufacturers can help avoid healthcare fraud by taking actions internally to prevent it.
6. UK Pharma Is Regulated by the MHRA
Medicines and Healthcare products Regulatory Agency (MHRA) regulates UK pharma companies. However, they have a significant role that goes beyond checking companies comply with regulations.
They guarantee that pharmaceuticals, medical equipment, and blood components meet safety criteria.
They also manage the safety and security of the supply chain for medications, medical equipment, and blood components.
The MHRA helps align worldwide standards and educate the public and healthcare professionals. They educate about the risks and advantages of medications, medical devices, and blood components. They support public-health-oriented innovation, research and development.
What You Need to Know About Pharmaceutical Companies
That’s a basic guide that tells you what most people don’t know or understand about pharmaceutical companies. You can share these facts, e.g. pharmaceutical systems solutions il, at a dinner parting or boast your industry knowledge at a pharma job interview. Either way, it shows you know what you’re talking about.
Did you find this article interesting? If so, make sure to check out our other posts for all things healthcare, business, finance, and more.
Infographic provided by The Emmes Company, a clinical research organization